所在位置:首页 > 贸促动态 > 市长国际企业家顾问会议 > 顾问报告 > 正文
健康北京,健康北京人——赛诺菲集团首席执行官魏巴赫
2012年05月29日 来源:中国国际贸易促进委员会北京市分会
赛诺菲集团首席执行官 魏巴赫
概要
能够受到北京市长邀请,提交这份关于进一步提高北京居民健康水平和药物可及性的建议,赛诺菲感到十分荣幸。由此,我们也感受到北京市领导对这一重要行业的长远战略眼光。北京市在2009年推出了“健康北京”计划,旨在推动北京成为一个具有国际水准的健康城市。借此机会,我们想首先为这一雄心勃勃的计划喝彩。
作为北京市政府的良好合作伙伴,赛诺菲承诺和整个医药行业一起,共同支持政府这一美好愿景,并致力于分享全球最佳医药卫生方案和尖端的生物技术,以帮助北京实现其目标。作为这一承诺的见证,赛诺菲迄今在北京的员工数已超过1000人,其中研发人员达200名。我们与顶级科研机构和优秀的生物科技公司携手探索,在药物研发的前沿领域联合开发创新型药物。2010年,赛诺菲荣幸地成为“北京生物医药产业跨越发展工程暨G20工程”成员企业。就在不久前,赛诺菲还被北京市经济和信息化委员会表彰为“2011年度生物医药产业突出贡献企业”。
提高医药卫生服务质量和控制成本,对实现北京市政府的目标有着重要意义,而创新药物在其中发挥着重要作用。梅肯研究院的研究显示,在20世纪,50%的经济增长源于医药创新和卫生设施的改善。此外,我们相信,经济增长和社会稳定携手并进,而它们都是创新的直接成果。
本报告包括了医药行业向北京市政府提出的一些建议,涉及如何提高优质医药卫生服务和创新药物的可及性,如何通过资源共享来培育和促进卫生服务和药物的创新优势,以及如何创造一个激励创新的宏观环境,进一步确保药物质量和安全。
我们相信,持续发展医疗卫生事业是北京市政府和医药行业的共同目标。这一领域目前有很多公共—私营合作的好机会,这些合作可以带来学术上、社会上、经济上的巨大影响。最重要的是,要把“健康”放回到医药卫生的核心。
我们再次感谢北京市政府的倾听,并希望双方可以就这份报告作进一步的讨论,以更好地支持“健康北京”的目标。
第一部分
北京在医疗与研发方面的成就和目标
1. 目标:“健康北京十二五计划”
北京市政府于2009年年末推出“健康北京:十二五计划”。采取的措施包括:全面整顿和改革公共健康服务;普及基本医疗;降低污染;使当地环境更加宜居,并确保居民生活更加安全和有保障。
该计划中设定的具体目标包括:人均寿命增长1岁;婴儿死亡率降至4%,孕产妇死亡率降至12.14/10万。
该计划还旨在:增强慢性疾病的防控能力;提供健康教育服务,促进居民生活方式转变;降低心脏病、恶性肿瘤等疾病的死亡率;提高医疗服务与保险水平;改善社区卫生服务;控制医疗费用,并降低个人支出比例。
为实现这些目标,北京市政府计划采取多项措施,如:
提高医疗服务水平:建立市级医院管理机构;医院管理与经营职能分离;扩大医疗资源;培训专家与专业人员;建设国际一流诊疗中心;培训社区和远郊区县医生。
控制医疗费用:增加政府支出;提高医疗保险水平;制定更快更好的报销制度;通过控制成本、投资政策、财务管理和药物采购来控制医疗费用结构,并推动医疗服务和药品定价体制改革。
管理药品安全:加强药品安全监督网建设;培育一千个药品质量诚信企业、发展一万名药品安全员,创建“药品放心城市”。
2. 北京在医疗改革、健康促进方面的努力及成就
尽管这是一个雄心勃勃的计划,但在各方努力下,该计划已取得重大进展。这些进展包括:
市政府已投入巨资来提高基本医疗服务能力。截至2010年末,所有卫生院已经转变为卫生服务站。已有约310个社区卫生服务中心在市区建立。根据媒体报道,北京市民对社区卫生服务中心的认可度已明显提高,社区卫生服务中心或服务站接待的就诊人数达6000多万,超过了大医院的就诊量。
平均寿命增加,孕妇死亡率开始下降,婴儿死亡率显著降低,达到了发达国家水平。传染病发病率也显著下降。
已经开展11项主要公共卫生计划(例如免费为妇女提供乳腺癌筛查)。政府为60岁以上和6岁以下市民免费接种流感疫苗,使北京市成为中国其他省市地区的榜样。
北京市在全国率先实现基本医疗保险的全覆盖,惠及老人、儿童和失业人口。
医院管理水平和效率正在不断提高,更多患者到社区卫生服务中心接受基本治疗。
整体安全得到了改善。重点关注食品和药品安全、产品质量、水安全、紧急情况或灾害管理等问题。
生活水平得到了提高。就业机会增加,个人收入上升,并且教育得到了改善。
第二部分
面临的挑战以及来自医药行业的建议
1. 健康服务的可获得性
a) 人口压力和非传染性疾病(NCD)的上升表明需要为公众和医护人员提供预防与教育计划。
2011年世界银行的一项调查显示,在40岁以上中国人群中,非传染性疾病的数量在未来二十年中将会翻倍,甚至达到三倍,其中大部分将出现在未来10年内。由于政府缺乏足够的应对措施,心血管疾病、中风和糖尿病预计将会给中国经济造成5500亿美元的损失(2005-2015年间)。鉴于中国人口老龄化与劳动力规模缩减的情况,这种巨大的经济损失增加了未来经济放缓的可能性,并将带来重大的社会挑战。北京市卫生局近日表示,癌症、心脏病和脑血管疾病已经成为北京居民死亡的三个主要原因,占总死亡人数的73.8%。非传染性疾病的快速增加源于两大潜在原因。首先是人口老龄化。根据2010年北京人口普查结果,65岁以上人口达171万,占8.7%,比2000年的114万人增长了49%。
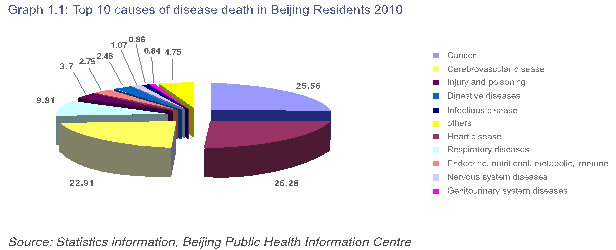
此外,快速工业化和收入水平的提高已经导致与污染、吸烟和整体膳食脂肪摄入增加等相关问题的增多,这个问题在北京更为明显。据瑞银的价格与收益报告显示,以纽约的净工资为100保持不变,北京的比较工资在2006年为10.9,2011年,这一数字已经攀升至16.3。名义上,北京农村居民2011年人均纯收入甚至达到了14700元人民币(2332美元),较2010年增长了约14%,扣除通胀因素,增幅约为8%。与此同时,住院费用自2008年以来平均每年上涨20%,这一上涨在很大程度上是被高血压、糖尿病等“富贵病”推动的。具体而言,在2010年,非传染性疾病占北京居民全部死亡人数的90.36%,其中癌症死亡率为158.73/100000人,心脏病死亡率为156.97/100000人。(有关疾病死亡原因的详情,请参考图1.1)
从经济意义上来讲,普通疾病尤其是非传染性疾病的上升导致了社会资源和支出的日益紧张。例如,在2008年,北京市医疗保险收入保费212亿元人民币,支出177亿元人民币;2010年,这一数字分别为296亿元人民币和285亿元人民币。如果这种趋势持续存在,在缺乏庞大政府补贴的情况下,保险基金将会在不远的将来面临入不敷出的窘境。

如前所述,北京市政府已投入巨资来提高其基本医疗服务能力。然而,尽管提升整体医疗基础服务是一个非常明确的首要任务,但劳动力短缺已成为一个重要挑战。根据2010年的估计,北京市实际需要社区医务人员3万人,缺口达1万多人。
关于提高医疗服务可获得性和预防水平的建议(代表中国外商投资企业协会药品研制和开发行业委员会“简称RDPAC,以下同” 和制药行业):
1) 进一步发展公共健康教育计划:在预防和治疗非传染性疾病方面,及早接种疫苗、疾病筛查、开展关于疾病治疗手段的教育等预防措施,和疾病管理一样至关重要。这是一个公私合作能够且已经产生显著影响的关键领域,尤其对制药行业而言。例如,赛诺菲支持卫生部下属中国健康促进与教育协会,于2011年10月在北京启动“心行动”。该活动旨在教育患者及其家人更好地进行疾病预防和管理。北京目前已有16家三甲医院成为“心行动”成员。活动自推出以来,已在北京组织近2000场讲座,共有3万多名患者参与并获益。
2) 医疗专业人员培训计划:公私合作伙伴关系还能够帮助改善基础医疗服务。制药公司通过与公共机构合作,可以为医生和医院管理提供培训,也能够提供私人资助的奖学金和助学金,以促进学生进入医疗行业。这些行动有助于实现那些尚未获得满足的社会需求,并实现政府的目标。例如,中国糖尿病综合管理项目(China Initiative of Diabetes Excellence)是卫生部、中国疾病预防控制中心、中华医学会糖尿病学分会、世界卫生组织合作中心国际糖尿病中心、梅奥医学中心和赛诺菲共同密切协作的成果。该项目2011年5月启动,将在未来5年内培训500名合格专业人员,并由他们培训近1万名社区医生,并帮助建立糖尿病患者小组。
b) 健康状况改善与能否及早获得高性价比的治疗直接相关
处方药物使多种疾病的死亡率显著降低。这些药物带来了巨大价值,延长人们寿命,提高工作效率并使人们享受健康生活。除改善和延长患者寿命外,正确使用药物还可减缓慢性疾病的进程,并避免产生昂贵的急诊室就诊、住院、医疗和手术费用,从而在降低医疗护理费用方面发挥着重要作用。
目前,中国患者等待新药的时间明显长于其他市场(参考图1.2)。中国患者获得新药物的时间比美国晚8年(参考图1.3)。
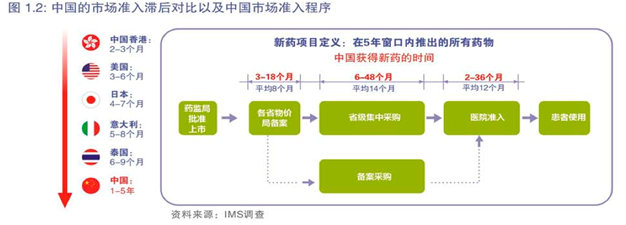

导致这种滞后的是四个主要程序:注册、定价、报销和招标。例如关于报销,在国家报销目录公布后,在省、市、地区级层面因当地要进行目录调整,患者获得可报销药物的时间因而被进一步推迟。例如,最新版国家药物报销目录于2009年11月公布,北京版本于2011年7月施行,报销目录的快速调整将会更快地给北京患者带来更好更多的医疗保障。
另一个滞后的原因是省市及地区药品招标制度,所有药品在被患者使用之前都要经过这一程序。(参考图1.2)具体来说,根据北京目前的体制,所有医院必须通过市级招标才能购买药物。然而,因为招标时间并不固定,许多新药错过了参加市级招标的机会,有时仅仅因为这些公司并不知晓正在进行招标。北京最近的一次招标于2009年举行,而上一次在2003年。
除市级招标外,还有两个非周期性采购渠道能让药物列入医院处方集,从而使患者能够获取这些药物,一个是药品招标备案程序,另一个是医院申请。然而,前者由于要在六个不同政府部门之间进行协调,实施难度较大因而很少被使用。
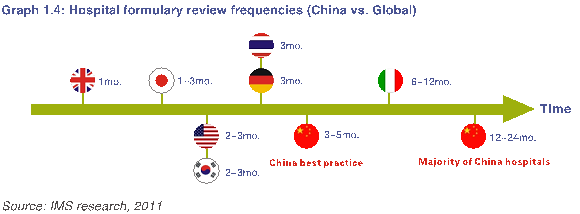
在医院层面上,不定期的医院新药审查会议也耗费大量时间,推迟了对新药的利用。具体来说,医院在药物种类和数量上的控制对临床医生提交新药物申请做出限制。此外,大多数医院没有定期新药审查会议,在进药审查时间表和日程上也缺乏方向性的要求。在中国,只有23%的医院每隔3到6个月举行一次定期新药审核会议。全球其他市场中的医院新药审查则更加频繁,如下图1.4所示。

因此,中国很多病人的治疗由于报销延迟以及缺乏创新药物而未能得到更好的治疗。
关于使病人及早获得高性价比治疗的建议(代表RDPAC和制药行业):
1) 在整个定价过程中为制药企业提供指南,必要时帮助这些企业与国家有关部门联络。这也将有助于促进本地制药业的成长,并使北京的患者能更早获取新药。
2) 加快北京市药品报销目录审查、调整程序,依据性价比、总体治疗负担和药品质量,对药品报销目录实施系统性审查和调整,这将确保患者能够及时获取新的救命药品。
3) 每年或每两年定期进行一次市级招标,并使各项要求实现标准化。如果是两年一次招标,建议在两次招标期间,每年有一次增补审查。不定期招标可能会导致不公平的市场竞争条件。例如,在当前体制下中标后,一些药品将会享有四年的竞争优势,而其他药品仅为两年。
4) 建立周期性的(每季度一次)和一贯的医院新药采购程序,以推动医院加快新药申请、审查周期,三级医院共享新药审查信息以提高整体效率。
2. 创新的价值
a)经济增长与药物创新直接相关
一项名为“造福社会的新药”的研究表明,创新药物在提高患者整体平均寿命方面发挥着关键作用。根据美国的一项调查(参考图2.1,此项调查建立在大量具有代表性的医疗服务提供者的样本之上)显示,在许多疾病领域内,例如肿瘤、糖尿病和传染病,有效使用创新药物可使患者生命延长0.8年。通过有效治疗,创新药物还有助于降低住院费用和急诊就诊量。
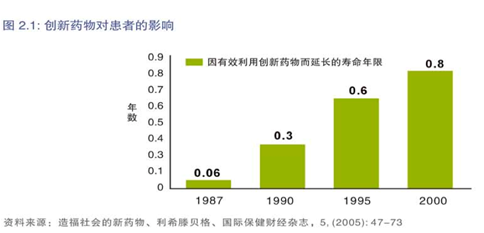
此外,根据梅肯研究院的一项研究,20世纪50%的经济增长得益于药物创新和卫生条件改善。换言之,由于人们的健康状态改善,他们能够为所在公司、工作和居住地的地方经济增长和发展做出更大贡献。鉴于创新药物在提高世界各地患者总体寿命水平方面所发挥的重要作用,在北京营造一个利于创新的环境显得尤为重要。
令人高兴的是,当今科学在治疗疾病等方面所取得的进展,超过了历史上任何时候。未来的趋势是发展更多更好的个体化治疗药物,从而为患者带来更加有效的治疗。塔夫茨中心(Tufts Center)进行的一项关于药品发展的研究显示,12%-50%的处于药品研发线中的药物为个体化治疗药物,同时,在过去五年中,生物制药公司对个体化药物的投资增长了75%。个体化药物能够为患有癌症、艾滋病病毒感染/艾滋病以及许多其他严重疾病的病人解决目前无法满足的医疗需求。通过避免治疗并发症的出现并确保患者获得最有效的治疗,个体化药物还可以帮助政府和社会有效应对医疗费用不断攀升的挑战。
然而,创新的全部价值只能随着时间的推移而逐渐显现。例如,从长远来看,通过有效治疗可以减少慢性非传染性疾病的并发症从而节约医疗费用。因此,北京将需要建立适宜的环境以谋取创新所提供的未来收益。
实现成本节约型创新的一条途径是,以增强型公私合作和建立伙伴关系的方式进行合作创新,这种合作方式也完全符合中央政府的期望和建议。赛诺菲自身的经验已经证明了这一事实。自2008年以来,赛诺菲已在研发方面建立了二十多项合作,包括在北京的七项合作。在短短四年时间内,这些合作项目取得了丰硕的成果,一个化合物正进入开发阶段,一个化合物待开发,另有三个化合物处于评估阶段。
关于通过更多资源共享以促进和培育药物与医疗创新优势的建议(代表RDPAC和制药行业):
1) 建立相关平台和激励措施,以进一步促进制药企业、大学院校和政府研究机构间的资源及经验共享。与学术机构建立伙伴关系,能够让企业的产品组合实现多样化并获得新技术,从而使研发过程更加有效。反之,大学和科研机构能够转化研究成果,充分实现其研究的市场潜力,同时为患者带来益处。
2) 开发相关平台,使跨国药企能够与政府资助的学术型研发机构共同工作、分享其先进经验,以帮助这些机构将科研突破顺利转化为可供患者使用的创新药物。例如,自2008年至今,北京肿瘤医院与赛诺菲之间的合作与专业经验共享,已经使北京肿瘤医院的一个单克隆抗体获得了两项专利,该分子对复发性肝癌有显著影响。在此基础上,赛诺菲目前正在进行两个概念证明试验,如果产生积极成果的话,在未来可能将挽救这种肝癌罹患者的生命。
3) 发展和培训人才,学术交流与公私合作伙伴关系将能为顶尖科学家提供更好的的培训。
b) 创新将为打造具有里程碑意义的健康首都铺平道路
提高医疗健康服务质量和价值(并控制其成本)对于实现“健康北京”这一目标至关重要,新药在实现这些关键目标方面发挥了重要作用。然而,政府药物定价政策对行业发展具有重大影响,并历来影响着药物的可及性和研发工作岗位的创造。

创新是昂贵的。创新药物研发的投资非常昂贵,并且涉及很高的风险。例如,赛诺菲在2011年将其总销售的14.4%用于研发。按整个行业的平均水平而言,研发中每花费1美元,只有0.70美元能够获得回报。总体而言,据估计,平均每个成功上市的新药所需的研发投资约为13亿美元。由于创新在改善人民健康和生活水平方面发挥了重大作用,因此,政府可通过调整药品定价政策以更好地促进创新药品准入,从而激励公司进行创新。
目前,在国家和北京市层面上,复杂而且很难驾驭的招标采购系统阻碍了创新动力,因为它对潜在的投资回报造成重大限制。与其他省市及地区类似,北京在国家发改委最高限价基础上,实行进一步降价以实现本市的降价目标,但并不保证销量会增加。因此,当要求大幅降低价格时,并未充分考虑创新激励。
安徽在2010年实施了一个类似制度,作为基本药物价格的控制手段。据了解,在某些极端的情况下,部分公司的中标价格甚至无法涵盖品牌公司为确保产品质量和患者用药安全投入的包装成本,更不用说创新成本了。
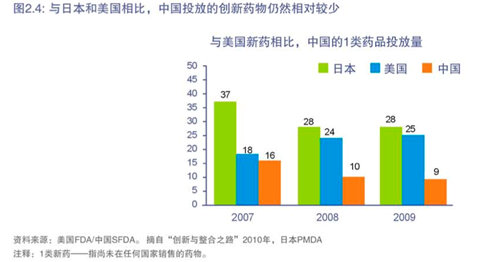
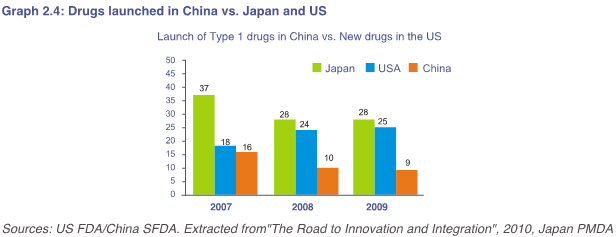
因此,尽管中国已经表明其创新的愿望,但与日本和美国相比,投放的创新药物仍然相对较少(参考图2.4)。
根据北京医药行业协会的统计,北京70%的药物制造商为中小型企业,每年的销售额不超过5000万元人民币,这意味着其目前的创新能力与北京成为中国生物制药行业领军者的雄伟目标之间仍然存在巨大差距。(资料来源:医药经济报,2011.12.26)
近几年,为了培育新药创新环境,北京市政府已经向相关学术机构和生物制药公司注入了大量资金,以支持其药物研发。除此而外,北京市政府还需进一步刺激这些公司追求自我创新的动力。
另一方面,一个良好的创新环境是指能够轻松实现信息共享、开展讨论并据此做出改善的环境。在这方面,北京能够并且应当在数据和信息共享方面实现全国领先,包括流行病学资料以及医院报告的不良反应事件。科研领域的信息、各种标准、法规等方面的信息不但可以和企业分享,而且还可以和科研机构分享,以促进研发工作。这样,北京能够构建一个集生物技术、制药企业和学术于一体的知识平台。
此外,如果实施正确的激励机制和政策,更好地保护知识产权,降低第三方利用专利获利的风险,外国和本地制药公司将能够在知识、技术的共享和转移中发挥更大作用。其他国家的经验表明,不断提高的知识产权保护水平可以积极推动更多企业在本地进行研发投资,从而提高国家的整体创新输出能力。
关于营造更好的创新激励环境的建议(代表RDPAC和制药行业):
1) 建立支持创新的定价制度:建立一个鼓励创新的定价制度,并确保公司有能力和资源在研发方面持续投资和再投资。
2) 为创新药物进入市级药品报销目录建立绿色通道。这将为北京患者带来益处,还将提高政府作为积极医疗投资者的形象。
3) 建立一个开放的数据共享系统,公布用于确定限价的数据参考和模型,这将有助于优化采购流程并实现公平定价。
3. 药品、疫苗质量与安全
a) 优先解决质量与安全问题
由于在中国销售的药品质量仍然存在着明显差异,药品质量这一未来医疗制度的核心原则,仍然是中国政府面临的挑战。
近年来,劣质药物一直是造成患者死亡和残疾的重要原因之一。2009年,多多药业双黄连注射液因不良反应造成三人死亡;2008年由黑龙江完达山药业生产的刺五加注射液因质量问题造成六人死亡;2007年因上海华联制药出现药物污染造成数百名白血病患者肢体残疾。
2010年9月,由毫无戒心的医疗机构购买的“救命”狂犬病疫苗,导致广西省来宾市的一个五岁男孩在接种一个月后死亡。狂犬病疫苗一直供不应求,不法分子看到了机会,通过销售假冒产品谋取高额利润。骗局被发现之前,已有1656人接种。在北京,尽管“质量”与“安全”备受重视,然而本市居民以及寻求在首都接受治疗的来自全国各地的患者的大量需求,导致疫苗和药物的需求越来越高,给了利益熏心的造假者可乘之机。2012年4月15日,中国国家食品药品监督管理局发出紧急通知,暂停销售和消费一系列报道遭受铬污染的胶囊。它们涉及13种药品,包括用于治疗感冒、肠胃问题和感染的药物,由国内九家制药公司生产,甚至包括个别知名企业。目前已经检测出问题药品的地区包括北京、江西省和吉林省。
2012年1月20日,国务院颁布了“十二五”计划,对提高药物安全性进行指导。该文件承认了目前许多关于药品质量的问题。这些问题包括中国制药企业较低水平的研发投资;与国际先进水平相比,一些仿制药存在质量偏差;药品营销不完善;定价与招标机制;一些制药企业过分强调经济效益;医生对药物的广泛不合理使用;猖獗的假冒药品,以及缺乏力度的药品监管执法。
解决上述问题的主要任务和项目包括:全面提高国家药品标准;通过技术改造鼓励制造商提高质量标准;加强药品全程管理;提高药品安全监测和早期预警标准;完善配套政策以确保药物安全;完善医药产业政策,在定价、招标采购和报销领域提供支持,使仿制药的质量具有国际先进水平;恢复药品安全问责制;为药品生产企业和相关专业人员建立信用评级系统;并在地方政府和官员的绩效考评中加入药品安全意识。
国务院要求地方政府领导实施这一计划,并将这一任务纳入到当地社会经济发展计划当中。国家食品药品监督管理局将在2013年中和2015年末领导开展中期评估和最终评估。
关于进一步确保药品质量和安全的建议(代表RDPAC和制药行业):
1) 制药行业和诸如赛诺菲等公司,能够并且愿意支持北京市政府不断完善安全和质量标准及程序,在建立不良反应报告、与卫生组织合作、以及与医疗专业人员和公众建立沟通系统等方面共享最佳做法,帮助北京成为一个模范卫生城市。
2) 用统一的质量标准区分药品的价值。建议采用9大关键质量要素模型(见附录)
3) 进一步加强培训与交流计划。培训和交流合作能够帮助公务人员更好地理解和建立国际化的标准。
4) 加强监督、检查和督导,以打击伪劣药品。在市政府的支持下,制药公司将努力促进行业内的自我规范,分享最佳实践,致力于改善整体监督机制。
b) 药品生产质量管理规范的实施与监督
2011年8月8日,国家食品药品监督管理局颁布了药品GMP认证措施。新的规范规定,国家食品药品监督管理局是监督全国范围内注射药物、放射性药物和生物制药的GMP认证工作的主导政府机构。此外,它负责监督进口药物的检验以及GMP检验的国际化。
该项措施规定,省市及地区级药物管理机构负责在其辖区内实施药品GMP认证,并应当建立GMP认证机构来开展相关的技术评估与现场检验。
到2011年底,5000个药品生产者中仅有154家通过了新的GMP认证。赛诺菲在北京的生产厂已于2012年4月份通过新版GMP检查。事实上,北京能够引领国家GMP的实施。除跨国公司外,北京的几家制药公司也成为这一领域的先锋。例如,赛科药业、双鹤药业、紫竹药业、以岭药业已获得美国GMP或欧盟GMP认证,这些活动将促进北京公司扩展海外市场。然而,北京食品药品监督管理局在2011年对80家药品生产商开展的一项调查表明,大部分药品生产商在实施新的GMP方面仍然面临诸多障碍。重要原因包括:
缺乏对新GMP的全面理解。尽管一些公司已经开始实施新的GMP,但他们并未完全理解一些先进的管理方法,或缺乏关于风险管理、变革控制等相关知识。这些公司中的大部分已经建立了管理程序,但尚未全面理解和设立目标。很多公司拥有独立的质量管理部门,但该部门却无法涵盖药品生产的全部周期,不能从源头上保障产品质量。一些企业并未理解新GMP的重要性,并且从未开展任何培训。
缺乏专业经验和受过良好培训的工作人员。根据同一研究,许多公司在质量控制方面严重缺乏专业知识和训练有素的工作人员。例如,一个公司在库存和生产管理方面的总人数为63名,但只有两个人(包括1名主管)负责质量控制。在某些公司中,管理团队从未接受或参加任何GMP相关的培训,并且很少关注雇员培训。中层管理团队也缺乏新GMP方面的综合知识。(资料来源:医药经济报,2011.12.26)
关于进一步加强GMP标准的实施从而与世界一流城市看齐的建议(代表RDPAC和制药行业):
1) 借鉴跨国公司的经验。北京市政府能够提供多样化的在线和离线GMP培训平台和计划。跨国公司可帮助引进和协调培训计划和平台。赛诺菲的技术专家曾于2011年在北京和杭州为当地食品药品监督管理局的检查员组织两场关于无菌生产和风险评估的培训,收效甚佳。
2) 成立工作组,鼓励本地的大型公司迈向更高标准,例如美国GMP与欧盟GMP。
c) 招标制度对药品质量的影响
为了解决安全问题,医疗政策需要考虑质量保证的复杂性,以确保生产企业能够对质量进行持续投资。然而,目前的定价与招标制度还需要在认可优质药品方面起到积极的引导作用(参考下面图3.1)。在省市及地区级招标时,首先对产品划分质量层次,从而影响价格的确定。一个重要挑战是,质量标准没有成熟的定义,新的创新药品的价值常常没有给予应有的考虑。例如,在采购谈判期间,对药品的选择主要集中在价格上,但很少考虑其他的重要因素,例如跨国公司遵守更高的药品生产标准,如欧盟的GMP,由此增强了药品的安全与质量。

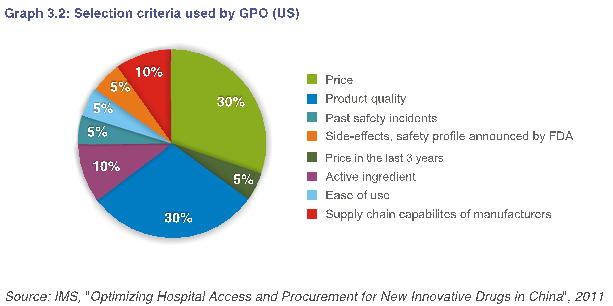
在中国,对质量提出的广泛要求,使人们难以区分质量上乘的产品。在其他市场,对质量问题的担忧较少,而对书面文件的要求能充分传达并标准化。例如,下图3.2中GPO(美国)所用的选择标准。
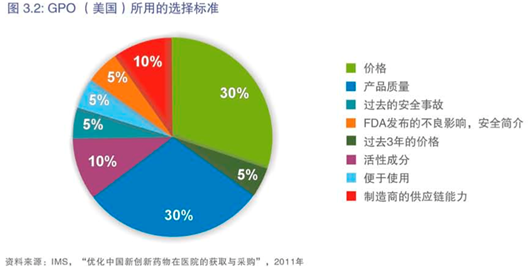
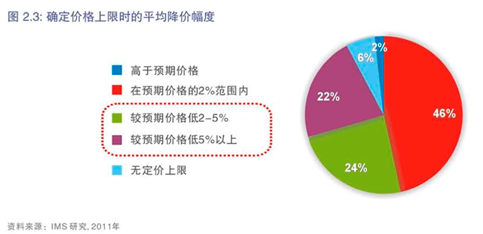
与其他省市及地区相比,在设定价格上限时,北京在质量认可方面做得较好。然而,有消息称,在不久的将来北京正在考虑制定一个更为价格驱动的政策,该政策要求在国家平均招标价格或全国最低招标价格基础上再削减10%。对此我们非常担心,我们希望在制定招标政策时更多考虑到产业的发展和北京的经济发展水平。
关于通过定价和招标制度调整认可创新价值的建议(代表RDPAC和制药行业):
1) 调整药品招标政策,赋予质量更高的权重;承认先进的GMP例如美国GMP和欧盟GMP的更高质量。
2) 根据质量差异、临床效果、成本效益、依从性优势而非仅靠价格驱动来选择药品。
第三部分
结束语
赛诺菲是北京市政府一个信守承诺、值得信赖的合作伙伴。赛诺菲会与医药行业共同支持市政府将北京市打造为与所有国际化都市比肩的国际医疗倡导者。作为一个多元化的全球医疗领导者,我们致力于分享全球最佳医疗解决方案和最新的生物技术以帮助北京实现其目标。
北京对高质量医疗卫生服务的需求猛增,得益于中国政府做出的各种努力,使北京人以及全中国人民的收入水平更上一个台阶。但是,随之而来的污染、吸烟和整体膳食脂肪摄入增加等问题造成人们的生活方式发生改变,并引发担忧。现在,北京市民对国际上的最佳创新药物需求渐增,他们应该获得安全、质量有保障的药品,更好更快地接受优质医疗服务。
北京政府卓有远见的战略计划旨在解决将来的健康问题,而且,在医疗方面的投资对拥有更好的前景至关重要。医疗投资可以在社会所有层面带来广泛、长期的收益,它会为经济、环境带来巨大利益,并且会实现更好的生活质量,促进社会稳定。投资健康可以提供三赢的最佳局面,使北京变成一个更宜居、更适合工作和投资的地方。
我们提交此报告,是为了协助北京政府实现其健康目标,并分享我们在这一领域中的专业知识。我们非常愿意与北京政府机构进一步探讨如何改善首都的医疗条件并提供更多有用的信息。
附录:9大关键质量要素
模型主体由9 大关键质量要素和34 个质量指标组成。展示如下:
Healthier Capital with Healthier Citizens
——Christopher A.Viehbacher,CEO,Sanofi Group
Executive Summary
Sanofi is honored to be invited by the Mayor of Beijing to submit our observations regarding the healthcare sector in Beijing. We would like to take this opportunity to first congratulate the Beijing Municipal Government, its leaders and administrators for their vision in this core sector. We applaud the launch of the ambitious program “Healthier Beijinger” in 2009, which aims to transform Beijing into an international healthcare champion on par with any of the world’s capitals.
As a partner of the Beijing Municipal government, Sanofi, together with the pharmaceutical industry, supports the government’s vision, and is committed to sharing global best-practice healthcare solutions and latest biotechnology advancements to help Beijing realize its goals. As a proof of this commitment, Sanofi currently employs more than 1,000 employees in Beijing, of which 200 are focused on R&D. We are collaborating with top scientific institutions in Beijing on discovery and co-development, and we continue to make strategic investments in our industrial operations. In 2010, Sanofi became a member of the Beijing Biopharmaceutical G20 Initiative. Most recently, Sanofi was recognized by Beijing Municipal Commission of Economy and Information Technology as one of the “Outstanding Biopharmaceutical Companies of 2011”.
Improving the quality and value of healthcare—and controlling its cost—are vital for the successful achievement of Beijing’s goals and vision. Innovative medicines play an important role in achieving these critical goals. According to a Milken Institute study, 50% of the economic growth in the 20th century was due to medical innovation and improvements in sanitation. Moreover, we strongly believe that growth and social stability go hand-in-hand and both are the direct fruits of innovation.
This report includes recommendations from the industry to the Beijing Municipal Government to enhance access to quality healthcare and innovative treatments; to promote and foster innovation in medicine and healthcare through greater resource sharing; to create an overall more motivating environment for innovation; and to further ensure drug quality and safety.
We believe that there is a shared goal between the Beijing government and the industry to continue to invigorate healthcare. There are great opportunities for public-private partnerships to make the greatest impact scientifically, socially, financially, and most importantly, put “health” back into healthcare.
We would like to thank the Beijing Municipal Government for its openness and hope this report can be further discussed to better support Beijing objectives.
Part 1: Beijing Achievements and Goals in Healthcare and R&D
1. Beijing’s Goals — “Healthy Beijinger: 12th Five-Year Plan”
Beijing Municipal Government launched “Healthy Beijinger: 12th Five-Year Plan” in late 2009. This ongoing program aims at developing Beijing into a first-class international metropolis in terms of boosting the level of health for citizens. Actions include completely overhauling and reforming the public health service; universal access to basic healthcare; reducing pollution; making the local environment more inhabitable and ensuring the lives of residents are safer and securer. Specific targets set out in the plan include: 1 year increase in the average life span; 4% decrease in infant mortalities and reducing pregnant women fatalities to 12.14 deaths per 100,000.
The plan also aims to: enhance the capability of chronic disease control; provide better health education in order to change harmful lifestyles; reduce heart disease and cancer-related deaths as well as deaths from other chronic diseases; upgrade the level of medical service and insurance; improve community health service; control health expenses, and decrease percentage of individual expenses.
In order to achieve these objectives, the Beijing Municipal Government has already defined a number of actions to:
Improve medical service: Establish a municipal-level hospital management institution; separate hospital management and operations; expand healthcare resources; train specialists and professionals; establish first-class level of international center for diagnosis and treatment; train community- and county-level doctors.
Control medical expenses: Increase government expenditure; upgrade the medical insurance level; develop a better and faster reimbursement system; control the medical expense structure by controlling cost, investment policy, financial management and medicine purchasing; and facilitate reform of medical service and drug pricing system.
Manage drug safety: Establish network for drug quality supervision; cultivate 1,000 trustworthy drug manufacturers with high-quality drugs; hire and train 10,000 drug safety supervisors, and become a City of Drug Safety.
2. Efforts and Achievements for Beijing on health reform, Healthy Campaign
Even though the objectives of the plan are very ambitious, major progress has been achieved. This includes:
The Municipal Government has invested heavily to enhance basic healthcare service capability, according to 2010 Beijing Development Briefing of Healthcare Resources and Medical Services. By the end of 2010, all county-level hospitals have been transformed into health service stations. About 310 community health service centers have been established in downtown areas. Beijing citizens are more aware of community health service centers, according to one media report. Basic health service centers or stations received more than 60 million visits. This number exceeded visits to big hospitals.
Average life span has increased, pregnant women fatalities have dropped, and death of infants has also decreased dramatically achieving the same level of developed countries. Prevalence of infectious diseases has also decreased significantly.
11 major public health programs, such as free breast cancer screenings for women, have been conducted. The government offers citizens over 60 and youngsters under six free flu vaccinations and this has set a standard for every province-municipal-region in China to follow.
Beijing was the first city in the country to achieve full coverage of basic medical insurance. Recipients include senior citizens, children and the unemployed population.
Hospital management and efficiency is improving and more patients went to community health centers for basic treatment.
Overall safety has improved. Focus has been on food and drug safety issue controls, product quality, water safety, emergency or disaster management etc.
Living standards have been boosted. Employment opportunities have grown, individual incomes are rising, and education has been improved.
Part 2: Remaining Challenges and Recommendations from the Pharmaceutical Industry
1. Access to Care
a) Population pressure and rise of non communicable diseases (NCDs) indicate the need of prevention and education programs for both public and healthcare professionals
According to a 2011 World Bank study, the number of NCDs among Chinese people over 40 will double or even triple over the next two decades. Most of these cases will occur during the next 10 years. In the absence of an adequate government response, CVDs, stroke, and diabetes alone are expected to cause a USD 550 billion loss to the Chinese economy (between 2005-2015). Considering China’s aging population and smaller workforce, such an enormous economic loss increases the likelihood of future economic slowdown and poses significant social challenges.
The Beijing Municipal Health Bureau said recently that cancer, heart disease and cerebrovascular disease have been the top three causes of death among Beijing residents, accounting for 73.8% of the total number of fatalities.
The rapid rise of NCDs is the result of two main underlying causes. The first is an aging population. According to the 2010 Beijing population census, the above 65 years old demographic among residents numbered 1.71 million, accounting for 8.7%. This marked a 49% increase over the figure from 2000, which was 1.14 million.
Additionally, rapid industrialization and rising income levels have resulted in a corresponding increase in problems associated with pollution, smoking and overall fattier diets. Nowhere is this problem more apparent than in Beijing. According to the UBS Prices and Earning Report, in 2006 New York net wages held constant at 100 compared to 10.9 in Beijing. By 2011 Beijing’s number had climbed to 16.3. In nominal terms, Per Capita Net income of even Beijing's rural residents reached over RMB 14,700 (USD 2,332) in 2011, up nearly 14% from 2010, or nearly 8% allowing for inflation. At the same time, hospital costs have risen on average 20% per year since 2008, propelled largely by the rise of “developed world” diseases, such as high-blood pressure and diabetes. Specifically, in 2010, NCDs accounted for 90.36% of all deaths among Beijing Residents, with cancer mortality at 158.73/100,000 persons and heart disease mortality at 156.97/100,000 persons. (Please refer to Graph 1.1 below for a full breakdown on causes of disease death.)
Economically speaking, the rise of diseases in general and of NCDs in particular, is placing a growing financial strain on social resources and spending. For example, in 2008 the Beijing Municipal Health Insurance received RMB 21.2 billion in premiums and paid out RMB 17.7 billion in costs. In 2010, the numbers were RMB 29.6 billion and RMB 28.5 billion respectively. If this trend continues, in the absence of large government subsidies, it is foreseeable that the insurance fund would be depleted in the near future.
As mentioned previously, the Beijing Municipal Government has invested huge amounts to enhance its basic healthcare service capability. Yet, while improving overall healthcare infrastructure is a clear priority, labor shortage has become a key challenge. Based on 2010 estimations, 30,000 staff is needed to adequately fulfill the needs in basic healthcare services. However, as many as 10,000 positions remain vacant and unfilled.
Recommendations to the Beijing Municipal Government on behalf of RDPAC and the pharmaceutical industry to improve healthcare access and prevention:
1) Further develop public health education programs: Preventative measures, such as early access to immunization and disease screenings, as well as promoting overall disease awareness on such topics as available treatment options and disease management are crucial in combating NCDs. This is one key area where public-private partnerships, especially in conjunction with the pharmaceutical industry, can and have already helped make a visible impact. For example, under the guidance of the Ministry of Health and the primary sponsorship of the Chinese Health Promotion and Education Association in combination with large support from Sanofi, the “Heart Action” was launched in Beijing in October 2011. The campaign is designed to educate patients and family members on prevention and management measures. Sixteen Class 3 hospitals in Beijing are currently part of “Heart Action”. Since its launch, “Heart Action” has conducted nearly 2,000 lectures in Beijing, reaching more than 30,000 patients.
2) Training programs for healthcare professionals: Public-private partnerships can help improve healthcare infrastructure. Pharmaceutical companies in cooperation with public authorities have provided doctor and hospital management training as well as offering privately-funded scholarships and fellowships encouraging students to enter the medical profession. These actions contribute to the fulfillment of unmet societal needs and the achievement of government goals. For example, the China Initiative of Diabetes Excellence is the result of closely coordinated joint efforts between the Ministry of Health, the Center for Disease Control, the China Diabetes Society, International Diabetes Center, Mayo Clinic and Sanofi. Launched in May 2011, the initiative aims to, within five years, train 500 qualified professionals who will then train nearly 10,000 community doctors and help establish diabetes patient groups.
b) Better health is directly linked to earlier access to cost-effective treatments
Prescription medicines have contributed to significant reductions in deaths from many diseases. These medicines bring great value, allowing people to live longer, be more productive and lead healthy lives. In addition to improving and extending life for patients, proper use of medicines also plays an important role in limiting health care costs by reducing chronic disease progression and avoiding expensive emergency room visits, hospitalizations, and medical and surgical procedures.
However, currently, the waiting time for patient access to new drugs in China is significantly longer than other markets (Graph 1.2). Compared to the US, patient access to medicines in China is delayed by up to 8 years. The delay is caused by lags in both launch and reimbursement (Graph 1.3).
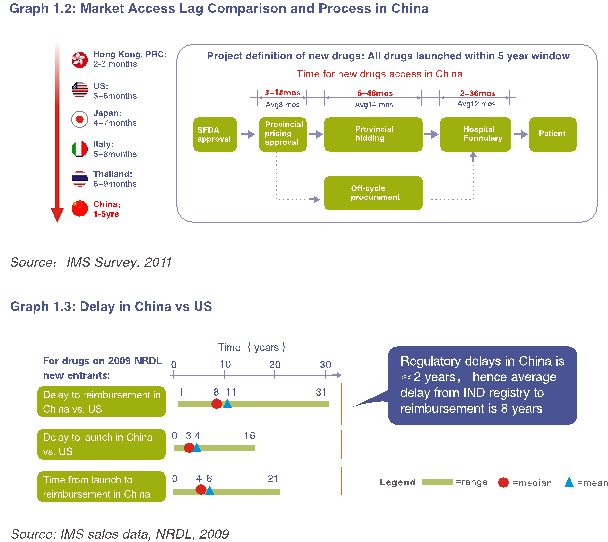
There are four major procedures, which lead to access delay: registration, pricing, reimbursement and bidding. Concerning reimbursement, after the national reimbursement list is published, patient access to reimbursed medicine is further delayed at the provincial-municipal-regional level due to local adjustment procedures. For example, while the new version of national drug reimbursement list was issued in November 2009, the Beijing version was issued in 2011, timely review of the reimbursement drug list would provide Beijing patients with enhanced health security.
Another hurdle is the provincial-municipal-regional drug bidding system, a process through which all drugs have to go through before having the chance to be used by patients (Refer to Graph 1.2 above). Specifically, under the current system in Beijing, all hospitals have to purchase drugs via municipal bidding. However, many new drugs miss the chance to participate in the municipal bidding due to the irregular timing of the bids, sometimes for the simple reason that companies were unaware that a bid was taking place until it was too late. For example, the most recent Beijing bidding was conducted in 2009, while the one before that was in 2003.
Aside from municipal bidding, there are also two off-cycle procurement channels through which drugs can become listed on the hospital formulary, thereby gaining access to patients. However, for request-based off-cycle procurement, the procedure is rarely used as it requires coordination between six different government bureaus.
At the hospital level, irregular hospital formulary review meetings are also time consuming and delay access to new drugs. Specifically, hospital control on the number of drug types restricts clinical physicians from submitting new drug requests. Additionally, most hospitals have no regularly scheduled formulary meeting and lack directional requirements on formulary review schedules and agenda. In China, only 23% of hospitals have regularly scheduled formulary meeting every 3-6 months. As shown in Graph 1.4, hospital formulary reviews in other markets are more frequent.
Therefore, the illnesses of a significant portion of the Chinese population may be under-treated due to delays in reimbursement and lack of access to new medicines.
Recommendations to the Beijing Municipal Government on behalf of RDPAC and the pharmaceutical industry for providing early access to cost-effective treatments:
1) Provide guidance to pharmaceutical companies in the complete pricing process and help companies liaise with national authorities when needed. This would help facilitate the growth of pharmaceutical sector and allow new drugs to gain quicker access to patients.
2) Facilitate the review/adjustment procedure for the Beijing municipal reimbursement drug list. Beijing could implement systematic reviews of the reimbursement drug list and adjust it based on cost-effectiveness, total burden decrease, and quality. This ensures timely access for patients to new potentially life-saving drugs.
3) Conduct municipal level bidding every year or every two years with regular timeline and standardized requirements. In between the bidding intervals, annualized off-cycle reviews are suggested. The irregular occurrence of biddings and off-cycle reviews lead to unfair market competition conditions. For example, under the current system, after winning a bid, some drugs enjoy the benefits for four years while others only two.
4) Establish frequent (quarterly) and consistent off-cycle procurement process to facilitate hospital formulary review cycle and formulary decision/review results sharing of Class 3 hospitals to improve efficiency.
2. The value of innovation
a) Economic growth is directly correlated with innovation in medicines
The Benefits to Society of New Drugs Study shows that innovative drugs have played a critical role in improving the overall life expectancy of patients. According to a US study (Graph 2.1), which is based on large, representative samples of healthcare providers, access to innovative drugs has extended the lives of patients by 0.8 years in many disease areas, such as oncology, diabetes and infectious diseases. Innovative drugs have also helped to reduce downstream hospitalization costs and emergency room visits through effective treatments.
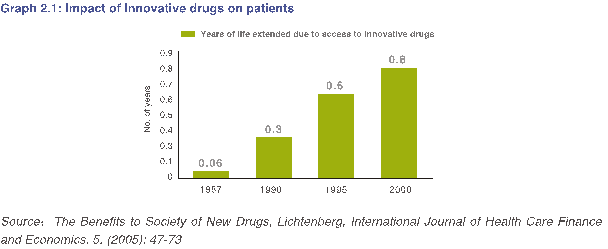
Additionally, according to a Milken Institute study, 50% of the economic growth in the 20th century can be attributed to medical innovation and improvements in sanitation. Because people are healthier and live longer, they can contribute more to the growth and development of their communities. Given the critical role innovative drugs have played in improving the overall life expectancy of patients worldwide, it is important for China and specifically Beijing to create a favorable environment for innovation.
What is more exciting is that science today holds more promise for progress against many diseases than at any time in history. The key future trends points to better and more personalized medicines, and therefore more effective treatment for the corresponding patients. A survey conducted by the Tufts Center for the Study of Drug Development found that 12% to 50% of drugs in the pipeline are personalized medicines and there is a 75% increase in personalized medicine investment by biopharmaceutical companies in the past five years. Personalized medicine offers enormous potential to address unmet medical needs of patients with cancer, HIV/AIDS, and many other serious diseases. It also holds the potential to help governments and societies meet the challenge of rising healthcare costs by avoiding treatment complications and making sure each patient gets the most effective care possible.
However, the full value of innovation can only be seen over time. The long-term savings of reduced complications of NCDs through effective treatment is a good example. Therefore, Beijing would need to set up the right environment in order to reap the benefits that innovation provides in the future.
One way to achieve cost-effective innovation is to innovate collaboratively in the form of enhanced public-private cooperation and partnerships. Moreover, this collaborative approach is fully aligned with the expectations and recommendations of the central government. Sanofi’s own experience attests to this fact. Since 2008 Sanofi has established more than 20 collaborations in R&D, including seven in Beijing. These collaborations have succeeded already in delivering, within a short span of four years, one compound to development, one ready for development, and three potential new candidates under evaluation.
Recommendations to the Beijing Municipal Government on behalf of RDPAC and the pharmaceutical industry for promoting and fostering innovative advances in medicine and healthcare through greater resource sharing:
1) Set up platforms and incentives to further promote the sharing of resources between the pharmaceutical industry and universities and government research institutions. Partnerships with academic institutions allow companies to diversify their portfolios and access new technologies that can lead to a more efficient R&D process. In return, universities and institutions gain the ability to capitalize on the market potential of their research through the transformation of research into commercial products, which also benefit patients.
2) Develop a platform for MNC pharmaceutical companies to further work with and share expertise in drug R&D with government-funded academic institutions to help them achieve breakthroughs in research into viable new innovative drugs for patients. For example, collaboration and sharing of expertise between the Beijing Cancer Hospital and Sanofi since 2008 has thus far resulted in the Beijing Cancer Hospital filing two patents on a monoclonal antibody that has an observable effect on recurrent hepatocellular carcinoma. Building from this, Sanofi is currently conducting two proof-of-concept experiments, which if yields positive results, might one day result in a life-saving treatment for patients with this type of liver cancer.
3) Develop and train talent. Academic exchanges and public/private partnership could further improve the training of the nation’s best scientists.
b) Innovation will pave the way for Beijing to be a landmark health capital
Improving the quality and value of healthcare—and controlling its cost—are vital for the successful attainment of Beijing’s “Healthier Beijinger” goals. New medicines play an important role in achieving these critical goals. However, government drug-pricing policies have a significant influence on the industry’s development, and have historically impacted patient access to medicines and creation of R&D jobs.
Innovation is costly. Investment in the development of innovative drugs also has high risk involved. For example, Sanofi spent 14.4% of all sales on R&D in 2011. On average, industry-wide, for every $1 spent in R&D, only $0.70 is gained in returns. Overall, it is estimated that it takes about USD 1.3 billion in R&D investment per approved new molecule. As innovation plays a large role in improving people’s health and standard of life, government drug-pricing policies can be adjusted to better promote the entry of innovative drugs thereby providing companies greater incentive to innovate.
Currently, on a national and Beijing-municipal level, a complex and difficult-to-navigate bidding and procurement system dampers the impetus for innovation as it places constraints on potential return on investment. Like other provinces-municipals-regions, Beijing is imposing further price cuts on top of NDRC or CIF prices to meet municipal price-cut targets, with no guaranteed increase in sales volume. Therefore, while significant price cuts are demanded, there has not been enough consideration placed on innovation encouragement.
When a similar system was implemented in Anhui in 2010 as a means of controlling the prices of essential drugs, it was observed that in the most extreme cases, the price set by small companies would not even cover the packaging costs, let alone the cost of innovation.
As a result, although China has stated its aspirations for innovation, there are still relatively fewer innovative drugs launched compared to Japan and the US (Graph 2.4).
According to the Beijing Pharmaceutical Profession Association, 70% of drug manufacturers in Beijing are SMEs with less than RMB 50 million in annual revenue, which means there is still a huge gap between current innovation capabilities and Beijing’s ambition of having a leading biopharmaceutical industry in China. (Source: Medical Economics, 2011.12.26)
The Beijing government has provided significant funding to academic institutions and biotech companies for new drug R&D in recent years, towards the goal of facilitating an innovative environment. Additionally, the Beijing government could work to further motivate companies to pursue self-innovation.
A favorable innovation environment is one in which information is easily shared, discussed and improved upon. In this area, Beijing could and should work towards leading the country in terms of data and information sharing, including epidemiological information and adverse events reported by hospitals. Information in scientific fields, standards, regulation, etc. can be shared not only within the industry but also with research institutions to facilitate R&D. Beijing can become the model of a knowledge-based industry platform gathering biotech, pharmaceutical industry and academia.
If the right incentives and policies are enforced to better ensure IPR protection, decreasing the risk that patented knowledge is used for-profit by a third party, pharmaceutical companies, both foreign and local, could play a bigger role in the sharing and transfer of knowledge and technology. Experiences in other countries prove that increased IPR protection is positively correlated with the willingness of companies to invest locally in R&D thereby increase a country’s overall innovation output.
Recommendations to the Beijing Municipal Government on behalf of RDPAC and the pharmaceutical industry for creating an overall more motivating innovation environment:
1) Create pricing system supportive of innovation. A pricing system could be created which encourages innovation and ensure companies have the capability and resources to keep (re)-investing in R&D.
2) A green channel could be established for innovative drugs to be included on the municipal reimbursement list. This will benefit Beijing patients and also boost the government’s image as a progressive healthcare investor.
3) Set up an open data sharing system. Publishing data references and models used for price ceiling determination can help optimize procurement and achieve fair pricing.
3. Drug/Vaccines quality and safety
a) National and Municipal priority to address quality and safety issues
Drug quality, a core tenet of the future medical system, remains a challenge for Chinese authorities because there are still significant quality differences among the drugs available in China.
In recent years, poor quality drugs have been responsible for deaths and disabilities of patients. In 2009 Duoduo Pharmaceutical’s Shuanghuanglian injections resulted in three deaths due to adverse reaction. In 2008 Ciwujia injection by HLJ Wandashan Pharmaceutical caused six deaths due to quality problems. In 2007 Shanghai Hualian Pharmaceutical's drug contamination caused limb disability of hundreds of leukemia patients.
More recently in September 2010, “life-saving” rabies vaccines bought by unsuspecting administrators led the death of a five-year old boy one month after inoculation in Laibin city, Guangxi province. Rabies vaccines had been in short supply and an “entrepreneur’ saw the chance to reap high profits by selling counterfeits. A total of 1,656 people were inoculated before the scam was discovered. In Beijing as well, though quality and safety are well-recognized values, the increasingly high demand for vaccines and medicines resulting from the needs of not only its own residents but also patients from all across China seeking treatment in the Capital, makes the city vulnerable against counterfeiters seeking quick profits. On April 15, 2012, the SFDA issued an emergency notice suspending the sale and consumption of a list of capsules with reported chromium contamination. They involve 13 kinds of drugs, including for the treatment for cold, stomach problems and infections, manufactured by nine domestic pharmaceutical companies including large, well-known ones like Xiuzheng Pharmaceutical Group in Jilin province. The problematic drugs so far have been detected in areas such as Beijing, and Jiangxi and Jilin provinces.
On January 20, 2012, the State Council issued the 12th Five-Year Plan directing ways to improve drug safety. The document includes admissions of many contemporary problems related to drug quality. These issues include low R&D investment by Chinese pharmaceutical companies; quality deviation of some generic drugs from advanced international standards; imperfect drug marketing; pricing and tender mechanisms; overemphasis on economic benefits by some pharmaceutical enterprises; widespread irrational use of drugs by physicians; rampant fake and counterfeit drugs, and weak drug regulatory enforcements.
Some of the major tasks and projects include: Raising national drug standards comprehensively; encouraging manufacturers to raise quality standards through technical renovation; strengthening the full process regulation of drug products; raising the standards of drug safety monitoring and early alerts; improving complementary policies for securing drug safety; improving pharmaceutical industry policies to provide support in the areas of pricing, tender purchase and reimbursement to generic drugs with quality at the internationally advanced level; reinstating drug safety accountability; establishing credit rating systems for pharmaceutical manufacturers and relevant professionals; and including drug safety awareness in the performance evaluation of local governments and officials.
Local governments are required to lead the implementation of this plan and integrate this task into their local social-economic development plans. The SFDA will lead the mid-term and final assessments in mid-2013 and at the end of 2015.
Recommendations to the Beijing Municipal Government on behalf of RDPAC and the pharmaceutical industry to further ensure drug quality and safety:
1) The pharmaceutical industry and companies like Sanofi can and are willing to support the Beijing Municipal Government to work on standards and processes of safety and quality. They can help Beijing become a model health city by sharing best practices in establishing adverse effect reports, cooperating with health organizations, and setting up communication systems with healthcare professionals and the public.
2) Unify quality standards used to differentiate value of pharmaceutical products. Consider using nine elements of quality as standards to differentiate products (Appendix 1).
3) Further pursue training and exchange programs. Public-private partnerships could also help officials to better understand and establish international standards.
4) Enhanced supervision, inspection and oversight could be considered in the fight against substandard medicines. Together with the support of the municipal government, pharmaceutical companies can work towards promoting self-regulation within the industry and contribute to the improvement of an overall supervision mechanism and the sharing of best practices.
b) Implementation and oversight of Good Manufacturing Practices (GMP)
On August 8, 2011, the SFDA issued Measures for Drug GMP Certification. The new regulations state that the SFDA is the lead government agency to oversee the GMP certification work nationwide of injectable drugs, radioactive drugs and biological products. In addition, it is responsible for overseas inspection of imported drugs and international harmonization of GMP inspections.
The measures provide that provincial-municipal-regional level drug administrative agencies are responsible for implementing GMP certification of drug products in their territories and should establish GMP certification agencies to conduct relevant technical evaluation and onsite inspections.
By the end of 2011, only 154 out of 5,000 drug manufacturers in China passed the new GMP. Sanofi’s manufacturing plant in Beijing has been inspected according to the new Chinese GMP in April 2012. The fact is Beijing could lead the national GMP implementation. Aside from multinationals, several Beijing pharmaceutical companies have also been pioneers in this arena. For example, Saike Pharmaceutical, Double-Crane Pharmaceutical, Zizhu Pharmaceutical, Yiling Pharmaceutical have gained US GMP or EU GMP certifications. These activities will facilitate Beijing local companies to expand overseas markets. However, 2011 research conducted by the Beijing FDA covering 80 drug producers in Beijing showed that most drug manufacturers are still facing obstacles towards the implementation of the new GMP. The key reasons identified include:
Lack of full appreciation of new GMP. Though some companies have started to implement the new GMP, they do not fully understand some of the advanced management concept and methodology or lack relevant knowledge about risk management, change control etc. Most of these companies have established management procedures, but these are not based on a full understanding of the objectives. Therefore, many of these companies have an independent department for quality management, but this department cannot cover the full cycle of drug production and cannot control the drug quality from the very beginning. These organizations do not understand the importance of new GMP and have never conducted any training.
Lack of expertise and well-trained working staff. According to the same research, many of the companies are facing a serious shortage of expertise and well-trained labor for quality control. For example, one company has a total staff of 63 for inventory and production management, but only two staff (including one supervisor) for quality control. In some companies, the management team has never received or attended any GMP-related training and rarely pays attention on employee training. The middle-management team also lacks comprehensive knowledge about the new GMP. (Source: Medical Economics, 2011.12.26)
Recommendations to the Beijing Municipal Government on behalf of RDPAC and the pharmaceutical industry to further enhance implementation of GMP standards on par with world-class cities:
1) It is recommended to leverage the experience of MNCs pharmaceuticals. Beijing Municipal Government could provide diversified GMP training platforms and programs, both online and offline. MNCs can help with establishing training programs and platforms as Sanofi did in 2011 by hosting two training sessions about Sterile Manufacturing and Risk Assessment. The two sessions were organized both in Beijing and Hangzhou to train local FDA Inspectors. The training modules were shared by 2 experts from Sanofi.
2) Set up a working group to encourage leading and large local companies to achieve higher standards, such as US GMP and EU GMP.
c) The impact of the bidding system on drug quality
To address safety issues, healthcare policies need to consider the complexity in quality assurance to ensure manufacturers continue to invest in quality. However, the current pricing and bidding systems are ineffective in recognizing quality drugs (refer to Graph 3.1 below). During provincial-municipal-regional bidding, products are first categorized into quality tiers, which impact price determination. A key challenge is that quality standards remain immaturely defined and the value of new innovative drugs is oftentimes not given due consideration. For example, drug selection during procurement negotiations is mainly focused on price, with less consideration given to other important factors such as MNCs’ adherence to higher drug manufacturing standards such as the EU’s GMP, which enhances assurance of a drug’s safety and quality.
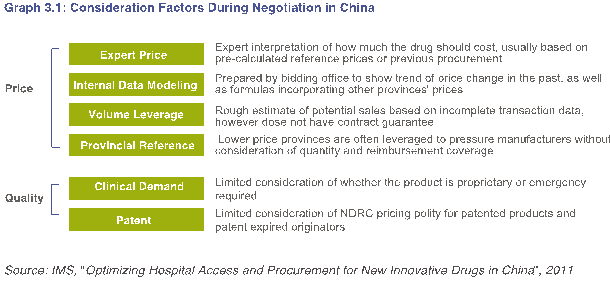
In China, broad requirements for quality make it difficult to differentiate products with superior quality. In other markets, quality issues are less of a concern and documentation requirements are well-communicated and standardized. For example, the selection criteria used by GPO (US) in Graph 3.2 below.
Compared with other provinces-municipals-regions, Beijing is doing better in terms of quality recognition when setting the ceiling price. However, there have been reports that Beijing is considering an even more price-driven policy in the near future, which would request a 10% further price decrease based on the average national bidding price or the lowest national bidding price. As such, we are highly concerned; we hope that other factors, such as the development of the pharmaceutical industry and Beijing’s own economic development, will be given more consideration when adjusting Beijing’s drug bidding policy.
Recommendations to the Beijing Municipal Government on behalf of RDPAC and the pharmaceutical industry for recognition of innovation through adjustment of pricing and bidding system:
1) Adjust the drug-bidding policy in which the weight of quality is higher; recognize advanced GMP such as US GMP and EU GMP as superior quality.
2) Select drugs based on quality differentiation, clinical effectiveness, cost effectiveness, compliance benefits etc. instead of only price-driven reasons.
Part 3: Conclusion
Sanofi has been, and will continue to be, a committed and trusted partner of the Beijing Municipal Government. Together with the pharmaceutical industry, Sanofi shares the government’s vision to transform the city into an international healthcare champion on par with any of the world’s capitals. As a diversified global healthcare leader we are committed to sharing the world’s best-practice healthcare solutions and latest biotechnology to help Beijing realize its goals.
Having been in China for three decades, Sanofi especially understands the soaring needs in Beijing for high-quality healthcare. Thanks to the efforts of the Chinese government, Beijingers and people all across China are enjoying income levels higher than ever before. But the resulting lifestyle changes associated with pollution, smoking and overall fattier diets are causes for concern. Beijingers are now increasingly demanding the world’s best innovative medicines.
Beijing Government’s forward-thinking, strategic plan targets future health problems and investment in healthcare is vital for a better future. Investment in healthcare has broad and long-term benefits across all levels of the community. It will bring significant benefits to the economy, to the environment and contribute to a higher quality of life that promotes social stability. Investment can provide the best conditions for a win-win-win situation to make Beijing a better place to live, to work and to invest.
We have provided this report in an effort to assist the Beijing Government achieve its healthcare goals and share our expertise in this field. We are very willing to meet with Beijing authorities to further discuss ways to improve health care in the capital and provide more useful information.
Appendix I : 9 Quality Elements
1. Framework of the model
A multi-factor assessment approach was applied during the design of the quality system incentive model.
Tier 1: includes nine quality elements, which are the common elements in a quality system.
Tier 2: includes 34 quality parameters, which can be applied in evaluating the respective quality elements (i.e. Tier 1).
主办:中国国际贸易促进委员会北京市分会
建设运维:北京市贸促会信息中心
京ICP证12017809号-3 | 京公网安备100102000689-3号


